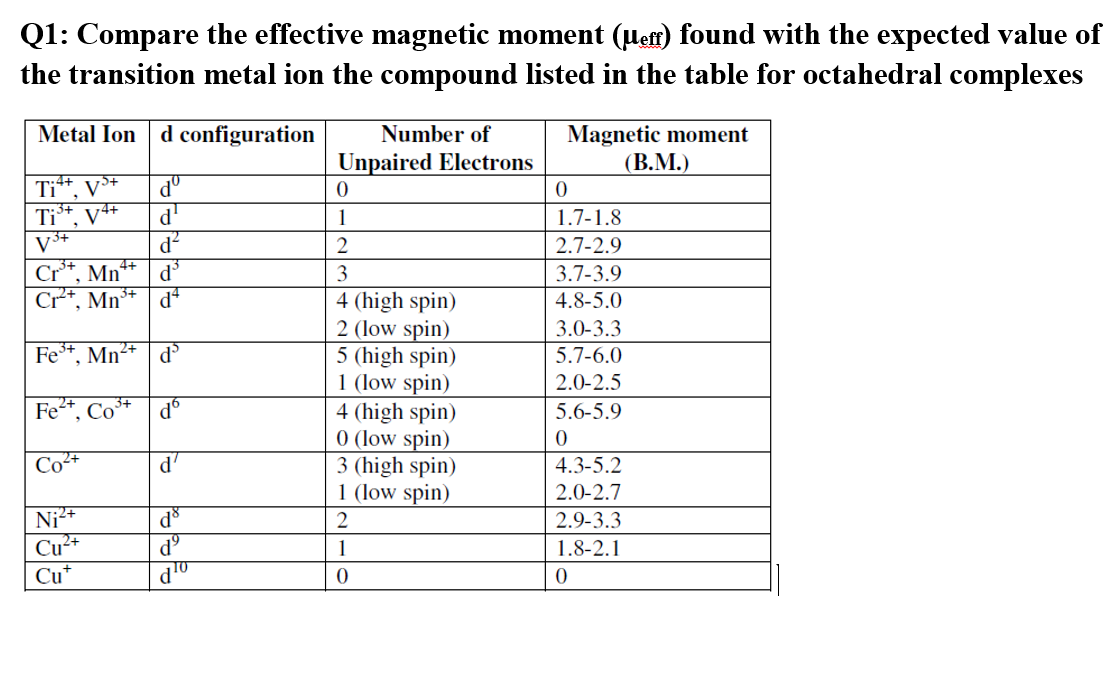
Accurate determination of the spin Hamiltonian parameters for Mn2+ ions in cubic ZnS nanocrystals by multifrequency EPR spectra analysis - ScienceDirect

Mn2+–Mn2+ Magnetic Coupling Effect on Photoluminescence Revealed by Photomagnetism in CsMnCl3 | The Journal of Physical Chemistry Letters
Calculate the 'spin only' magnetic moment of M2+ (aq) ion (Z = 27). - Sarthaks eConnect | Largest Online Education Community

How many unpaired electrons are present in Mn^(2+) ion ? How do they influence magnetic behaviour of Mn^(2+) ion?
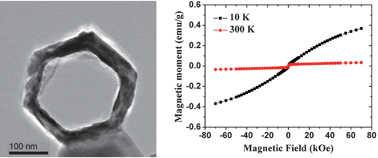
Single-crystal like mesoporous ZnO:Mn2+ nanorings of high optoelectronic quality formed by self-assembly of nanoparticles in an ultrasonic hydrolysis process - Nanoscale (RSC Publishing)

calculate the number of unpaired electrons in Ti3+ , Mn2+ and calculate the spin only magnetic - Brainly.in

Among V(Z = 23) , Cr(Z = 24) , Mn(Z = 25) and Fe(Z = 26) , which will have the highest magnetic moment?

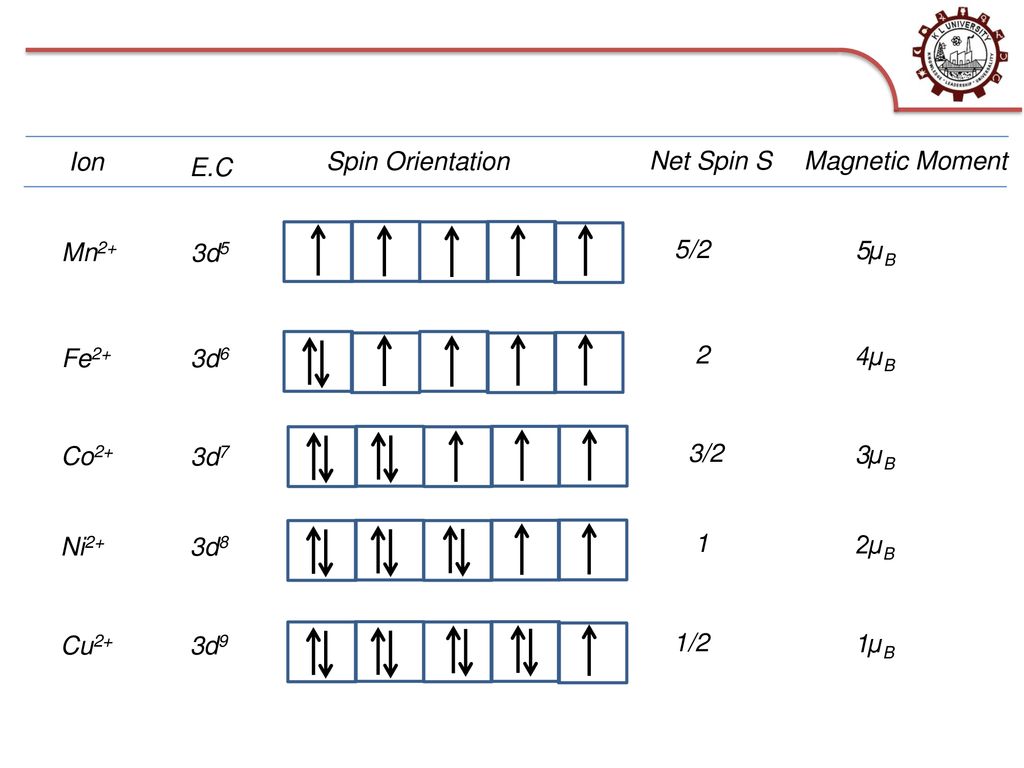






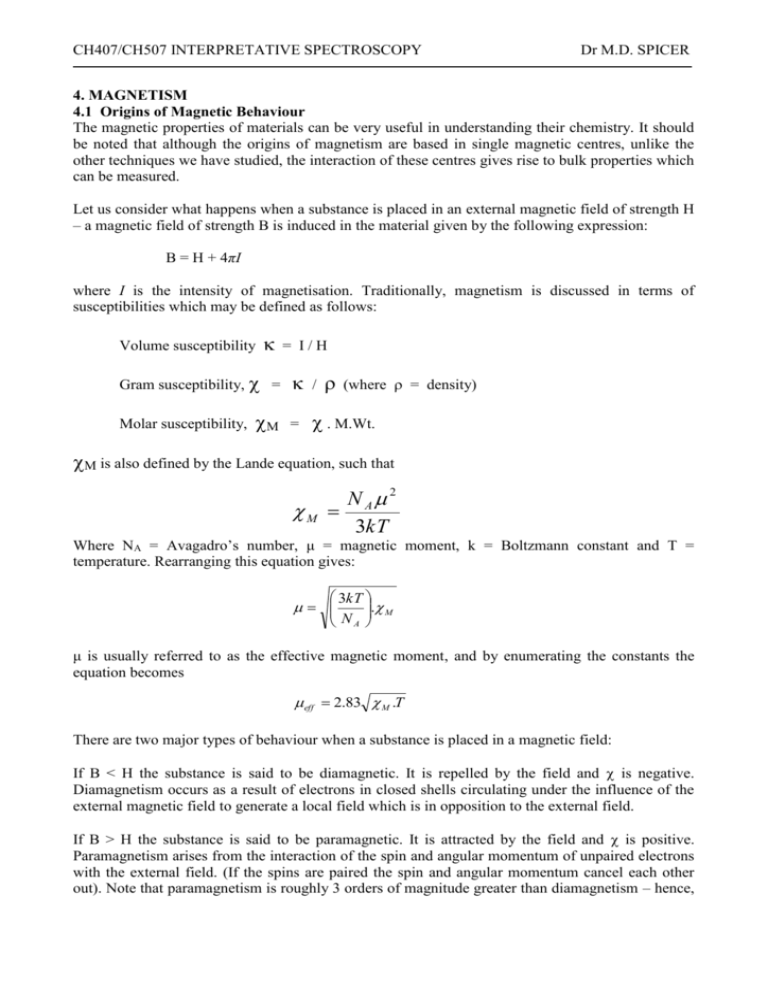



![Calculate the magnetic moment of Mn^(2+) ion. [Atomic number of Mn=25] Calculate the magnetic moment of Mn^(2+) ion. [Atomic number of Mn=25]](https://doubtnut-static.s.llnwi.net/static/web-thumb/643905480_web.png)